Raun Kupiec is Senior Vice President, Head of Global Regulatory Affairs at Vifor Pharma.
Vifor Pharma is a global leader in iron deficiency, nephrology and cardio-renal therapies and strives to help patients around the world with severe and chronic diseases to lead better, healthier lives. Headquartered in Zurich, Switzerland, the company has an increasingly global presence and a broad network of affiliates and partners around the world.
OXYGY: Why is being more agile a priority for you and your function at Vifor?
Raun Kupiec: As we increase the number of partnerships we have globally; and, at the same time build a new internal pipeline, we need to adapt the organization to be both flexible and efficient, multifaceted. This is part of preparing for whatever growth the future brings for Vifor, and whatever changes the Health Authorities may decide to implement to the regulatory framework. This requires agility from the department; meaning the ability to focus on different aspects of the regulatory framework while at the same time supporting a growing portfolio of therapies, products and solutions.
Where do you see Regulatory contributing most to the success of Vifor?
There are three imperatives: First, help answer the question ‘what do we have to play with’; namely, helping develop products and manage them through their lifecycle. Second, ‘where do we play’; helping expand and differentiate the company’s product portfolio both geographically and also therapeutically. Third, ‘permission to keep playing’; in other words, maintaining regulatory compliance under all the relevant frameworks.
What steps have you taken to be more agile as a Regulatory Affairs function?
The key is to align resources and capabilities to better focus on the business outcomes that Regulatory can help achieve. We have aligned ourselves along the following main concepts: our key therapeutic areas, labeling and clinical support, chemical manufacturing and controls (including formulation development and manufacturing improvements) and a center of excellence that ties all of the above together behind specific strategies while driving efficiency and building capabilities.
How do you cope with the relentless burden to deliver that we all face today?
You have to have some capacity that you can quickly deploy to provide focused support for major activities and periods of peak resource requirements across the department. We are developing a group of project managers within the Center of Excellence that can provide that function alongside our international strategy, life-cycle management and infrastructure teams.
It is said that a function is only as good as its people. Do you agree?
Partly. If you put a good person into a bad process, the process will win. In other words, you need well designed, efficient processes and structures that encourage collaboration both within the department and with stakeholders outside the Regulatory function, as well as the right attitudes, knowledge and skills.
Say more about the attitudes, knowledge and skills you look for in your broader team.
It is a question of mix, having the right balance for the roles that each of us plays in the global function. For example, a Regulatory product manager supporting a therapeutic area as member of a global brand team has to be a strategic thinker, providing insights and shaping product strategy to help maximize the value of that asset. At other times, or from other functions within Regulatory, what may be needed is to draft technical documents, which requires the ability to write with clarity and precision, and an organized command of details and data. We started with the basic RAPS framework (*) for regulatory affairs competencies, and then adapted and filtered. The key capabilities we have identified as most critical for Vifor’s Regulatory function are:
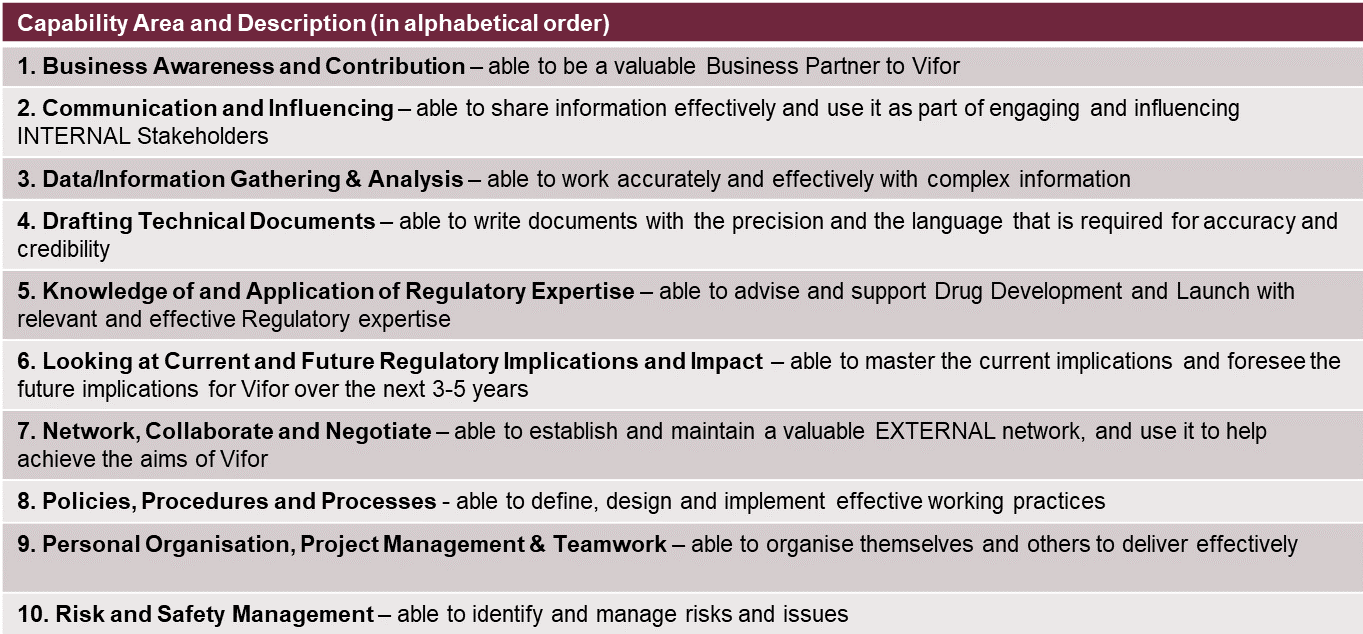
These have been translated by level of mastery into roles and job descriptions
In practical terms, how has that helped?
Back to agility, I want people to define themselves by their capabilities, not by the specific job they are performing today. Likewise, advancement is based on one’s ability to learn and demonstrate specific capabilities. Every person evaluates themselves against demonstrable behaviors, gets feedback from their manager and identifies how best to develop themselves.
How honest are people in their self-evaluations?
We have learned that it is important to stress that being brutally honest is the best way to get the resources and opportunities a person needs to grow and develop. We pay special attention to cultural differences knowing that there is a tendency in some countries to be more self-critical and in others to see evaluation as a chance for self-promotion. We are getting better at managing the nuances. Whatever the baseline, the key is for managers and their direct reports to clarify what is expected, where the development opportunities are and how to get better.
Who defined what the expected capabilities should be?
We involved a cross-section of the entire department to build ownership for the behavioral descriptions of what is expected by position. We took an outside-in approach building on input from leading pharma companies, got input from our business leaders and our own subject matter experts developed a framework that is forward looking, aligned with our vision and mission as a function and company. Since they developed it, our managers see the value in using it for professional development, including their own.
What advice would you give others embarking on a similar journey to elevate the Regulatory function in their organization?
It really takes time, effort and focus. To make it a success, you need to engage your leadership team and in turn, their direct reports. It is important not to forget the iterative nature of building capabilities. Trying to achieve perfection in the framework and mapping is not a productive use of the time. Don’t get lost in a numerical exercise of where you are and where you want to be. Take the first steps, and then adjust and grow as you develop. This is about creating a new, agile dynamic in the organization that is business and improvement focused.
(*) RAPS, Regulatory Affairs Professionals Society. https://www.raps.org/